No matter how big or small the water drops are, one thing common is that they are spherical when placed on flat surface. Whether you see it in the form of dew on grass or droplets of water tipping from the tap, they come in spherical shape. Have you ever wondered why is that so?
If we look closely at water droplets then we know that a drop of water consists of numerous water molecules and there exist a week attractive force between water molecules. If a single molecule is within the liquid water then it is surrounded by other water molecules in which case the single molecule will experience an attractive force from all directions. Hence there would be no net force on that water molecule.
Understanding the Surface Tension of Water Molecules
However on the surface of water, the water molecule will experience intermolecular attractive force from the sides as well from the molecules below the water surface. Since there is no attractive force above the surface of water therefore the water molecules on the surface will experience force of attraction in a direction which is tended towards center. Since all the water molecules on the surface face are pulled in the direction of center that is why water droplets are spherical in shape.
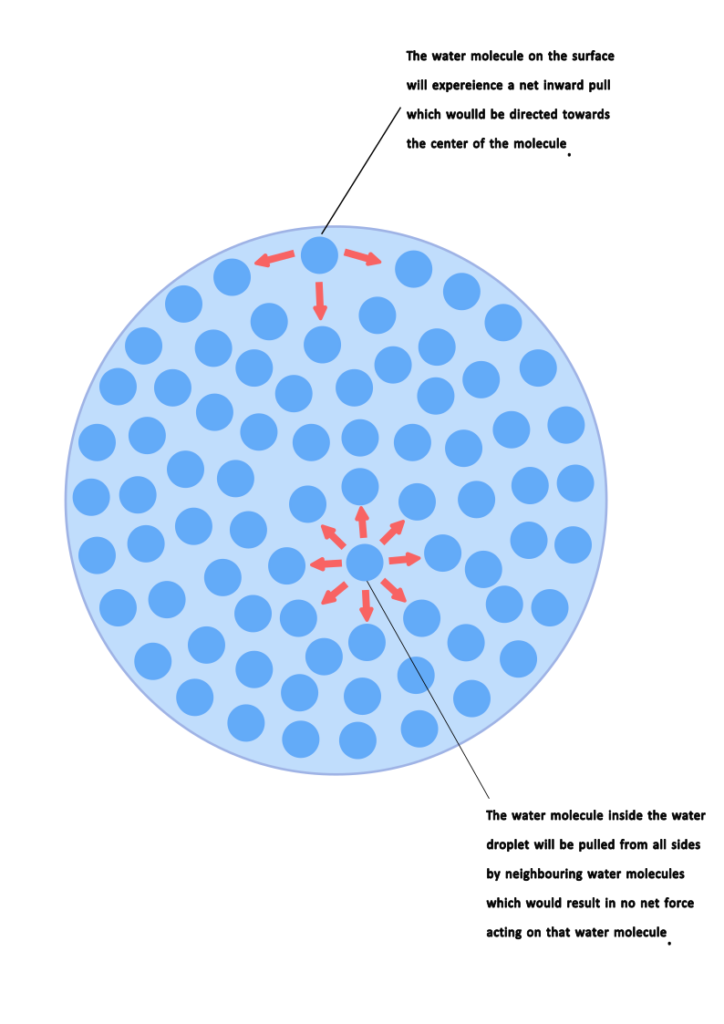
The tendency of liquid surface to occupy minimum area possible due to the intermolecular forces is known as Surface tension.
The spherical characteristics of water droplets can be explained by Surface Tension.
Surface tension is a physical property of liquids that describes the elastic tendency of a liquid’s surface, which makes it acquire the least surface area possible. It arises due to the cohesive forces between liquid molecules. In the case of water, the molecules at the surface experience a net inward force because they are only attracted to the molecules beside and below them, not above (since there’s air above). This results in a “skin” on the water’s surface that can resist an external force.

